PHYSICIAN, ALLIED HEALTH PROFESSIONAL, OR HEALTH SYSTEM CEO; YOU MAY BE THE DOJ #1 TARGET
False Claims Act (FCA), Coding – falls into:
“fraud (intentional)” or “abuse (innocent mistake).”
*
The #1 Target that the DOJ Criminal Division will prioritize and investigate begins with Healthcare and Procurement Fraud—memorandum: May 12, 2025.
A
What was once considered criminal, in some cases, is now considered Civil.
Don’t take any chances. Legal Representation is a must to avoid turning a civil financial penalty into a criminal sentence.
Example of Criminal Fraud (Prison Time),
With restitution Payments of over $6 M, the reality is that after prison, the ability to make those payments in full is less than likely.
A
A
S
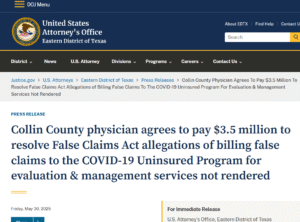
Example of Civil (No Prison – Pay Us):
Physician agrees to pay $3.5 million to resolve False Claims.
A
In both cases, Legal Representation is a must. If it becomes a criminal charge, the nuances here may extend beyond the traditional legal defense. Once a criminal charge is made, judges are very interested in understanding what happened in your life that brought you to their courtroom today. Help them answer that question. Unsure? Ask me!
In the AMA’s article on coding, I have highlighted some of their recommended examples.
A
A
*
Questions? Call Today,
Physician Presentence Report Service
Dr. Blatstein
240.888.7778
PPRSUS.com
WHITE-COLLAR, SENTENCE MITIGATION SOLUTION
*
*
Here is a deeper dive.
An opinion article in the Journal of the American Medical Association features an essay by Martin Gaynor, a professor at Carnegie Mellon University’s Heinz College of Information Systems and Public Policy. Gaynor argues that major U.S. health care companies have evolved into comprehensive “big health care” platforms, merging roles of insurers, providers, pharmacies, diagnostics, and essentially all aspects of care, including data analytics. He warns that this trend threatens to worsen the existing monopoly power issues in the U.S. health care system.”
The adverse effects of platformization include making it harder for people to access healthcare. It also alters competition in ways that make it difficult for smaller companies to survive. There are conflicts of interest that lead to the disregard for or exploitation of regulations. Additionally, large systems dominate the market, limiting competition and making it challenging for independent doctors, practitioners, and pharmacists to start and grow their businesses within their communities. This situation also stifles innovation.
Health care and life sciences companies will continue to be under the microscope and should be aware that:
-
- Investigation and prosecution of health care violations dominate the enforcement list for the Criminal and Civil DOJ.
The DOJ has expanded the types of reports eligible under its Corporate Whistleblower Program to include information on potential violations involving public healthcare benefit programs.
a
The Plan Specifically Targets Health Care
The Plan highlights “fraud that endangers consumer health and safety,” focusing on violations of the Controlled Substances Act and the Federal Food, Drug, and Cosmetic Act. This includes illegal fentanyl-laced counterfeit pills and unlawful opioid distribution by medical professionals and companies.
…these priorities likely signal a sharpened focus on interactions involving federal healthcare programs, patient support programs, interactions with healthcare professionals, and the distribution and manufacture of drugs and devices.
Key Enforcement Priorities
Waste, fraud, and abuse of public funds: Cases involving fraud against Medicare, Medicaid, COVID-19 relief programs, or defense procurement contracts remain a top concern, particularly where conduct threatens the integrity of federal programs.
a
Emphasis on Self-Reporting
The DOJ also updated its Corporate Enforcement and Voluntary Self-Disclosure Policy (CEP) to offer more certainty and incentives for companies to self-disclose misconduct.
A
*
What This Means for Companies
In response to the DOJ’s updated priorities, companies should reassess their compliance programs, strengthen reporting systems, and focus on early detection to mitigate liability.
Assume nothing and anticipate ongoing revisions to the Department of Justice (DOJ) policies. Therefore, large hospital systems or individual practices need to integrate corporate governance into their best practices. Keeping your policies up to date, informed by counsel, your local or national associations, or even malpractice carriers, will be invaluable. This proactive approach ensures that you have current policies in place in the event of an unexpected visit.
A